治験サプライチェーンサービス(治験薬供給・治験薬製造)
全ての治験にグローバル対応の治験薬供給ソリューションを
あらゆる治験の中心には、治験薬がスケジュール通りに無事届くのを待つ患者さんがいます。バイオ医薬品企業各社は、激しい新薬開発競争の中で、コスト、時間、品質のバランスを取りながら、治験と治験に参加している患者さんに最良の成果を提供するという課題を解決しなければなりません。
パセオンのグローバル治験サプライチェーンサービス(旧称:Fisher Clinical Services)は、世界各国の臨床研究と患者さんへの貢献に真摯に取り組む人材が提供するサービスです。30年以上にわたる治験関連サービスの経験と専門知識を駆使し、従来の低分子医薬品、バイオ医薬品、先進治療などあらゆる治験薬について、サプライチェーン全体を強力に支援いたします。
サービスの幅の広さ、深さ、柔軟性でサポートいたします。
30年以上にわたる治験サポート経験と幅広いサービス、専門知識を備えたパセオンは、サプライチェーン全体を強力に支援することが可能です。
治験関連サービス・ソリューション・技術力
世界各地のバイオレポジトリーを結ぶネットワークで、膨大な数の検体の完全性を維持してきた経験を駆使し、特殊な生体試料の室温〜低温輸送、保管、処理に対応します。各バイオレポジトリーにおいて同等の設備・体制を整備し、処理量や地理的位置にかかわらず、治験の要件を的確に満たし、変動を最小限に抑えます。私たちの活動の根幹には、規制要件、業界のベストプラクティス、高度なトレーニングを積んだ人材を基盤とする総合的かつ統合されたグローバル品質システムがあります。
バイオ医薬品の管理・保管
ワクチンや個別化遺伝子治療製品の製造、将来の創薬のための細胞株の保護などあらゆる状況において、パセオンはお客様のかけがえのない生体試料を守ることに全力で取り組んでいます。
- 専用施設、液体窒素輸送車両、ロジスティクス専門家のグローバルネットワークを駆使した室温〜低温輸送、保管管理サービスを提供いたします。
- 在庫管理システムにより、お客様はデータや在庫情報に毎日24時間アクセス可能です。
- 保管装置には、温度逸脱を防ぐため、複数のコンプレッサーが備わっています。また、毎日24時間、監視し続けるモニタリングシステムも設置されており、温度逸脱が発生した場合には担当者に通知が送られます。
- 侵入検出アラートを含む包括的なセキュリティ・保管品追跡システムが整備されており、施設、冷凍庫等の低温保管装置はアクセス専用のカードリーダーによりアクセス管理が行われています。
- すべての施設には無停電電源装置(UPS)と1つ以上の補助発電機が設置されています。
- 検体処理技術
低温ロジスティクス・流通
世界の戦略的拠点を結ぶ業界最大の自社cGMP施設ネットワークにより、生体試料、細胞株、単回投与細胞治療薬の保管・輸送を支援いたします。適格性の確認された幅広いソリューションにより、シングルユース、高性能再使用可能システム、少量輸送、単独バルク輸送、マルチパレット輸送などお客様のニーズに的確に対応いたします。
- 国際航空運送協会(IATA)および危険物質取扱い(HAZMAT)のトレーニングを受けたスタッフが温度管理下での出荷作業(-70℃、-20℃、2〜8℃、15〜25℃)を行い、規定環境外の時間を最小限に抑えます。
- Good Distribution Practice(医薬品の物流に関する基準)(EudraLex Annex 15、EU GDPガイドライン、21CFR211.142および21CFR211.150)の要件遵守の確保
- 低温輸送品のための共用/専用ドライシッパーなど、能動的/受動的に温度を維持する適格性確認済みの自社輸送システムにより、あらゆる温度・気候条件に対応いたします。
- 英国・EU向け品のQualified Person(QP)による出荷
- ジャスト・イン・タイムの包装・ラベリング・流通サービス
- 設備・測定機器はNIST(米国標準技術研究所)基準に従って校正されており、出荷時の適格性評価試験を通過しています。
- 欧州、米国、中国向けの環境に配慮した搬送容器返却・再使用プログラム
連続モニタリングソリューションによる低温輸送品の輸送状況の見える化
- 専門輸送サービスにより、現在位置、配送品温度、デュワー瓶の向きなど重要パラメータを毎日24時間、追跡・モニタリングいたします。
- 携帯電話からアクセスできる管理されたウェブポータルにより、リアルタイムでの輸送状況と温度データを治験実施医療機関に提供。
- 21 CFR Part 11に準拠した温度報告書やバリデーション済みのデータロガーを備え、必要な時にすぐ対応できるジャスト・イン・タイムの輸送体制
専門ロジスティクスのエキスパートチームと適格性が確認された輸送業者のグローバルネットワークにより、最高水準のサービスを提供いたします。
- 輸送方法の最適化
- 低温物流・市販輸送経路バリデーション
- 通関・規制対応サポート
- 発送サービス
二次包装・ラベリング
超低温でのラベリングは非常に難しい作業ですが、パセオンはお客様との共同作業により、温度要件、さまざまな投与方法、盲検化要件、各患者さんの情報を考慮した一次・二次ラベリング戦略を構築いたします。また、パセオンは商用包装の実績も誇ります。
- Drug Supply Chain and Security Act(医薬品サプライチェーン・セキュリティ法:DSCSA)要件およびFalsified Medicines Directive(偽造医薬品対策指令:FMD)を遵守したシリアライゼーション(シリアル番号の割当て)設備・ソフトウェア
- すべての包装・ラベリング作業を適切な温度下で実施
- 時間制限のある発送プロセスにカスタマイズしたジャスト・イン・タイムの包装・ラベリングワークフロー
- 翻訳・規制対応サポート
- ラベル・構成部品の印刷とバリデーション
採取・投与キット
採取中に汚染される検体は、平均13%にのぼります。キットを用いることにより、汚染リスクの低減と採取プロセスの標準化を実現でき、分析前の変動の抑制に大きく寄与します。パセオンが提供する採取・投与キットは、治験での検体採取や治験薬投与時に必要なものがすべて揃っており、採取から投与/分析まで検体取扱いのあらゆる側面の標準化に貢献します。
検体処理
最先端のワークフロー、高度なゲノミクス手法、そして包括的な情報科学ソリューションを駆使し、バイオセーフティレベル2(BSL-2)および封じ込めレベル3(CL3)のラボにおいて保管検体を迅速かつ正確に分析します。
検体処理技術の内容
- 血液・血液由来物質[血清、血漿、赤血球(RBC)、末梢血単核細胞(PBMC)、バフィーコート、血塊)、便、尿、唾液、脳脊髄液(CSF)、その他体液、癌組織/細胞株、セルフリーDNA(cfDNA)、ホルマリン固定パラフィン包埋(FFPE)ブロック、羊水、臍帯血、胎盤、母乳、胎便、切り取った爪、毛髪、精液、子宮頸部スワブ、細菌、植物、食物、土壌、ウイルス、その他のヒト/動物検体などさまざまな検体に対応いたします。
- PBMC抽出、凍結保存、生存率試験
- 自動検体処理、分注、バイアル施栓/開栓、ラベリング
- 自動血液分画(バフィーコート抽出)
核酸/タンパク質の自動抽出および分析
- さまざまな検体からのcfDNAやゲノムDNA(gDNA)、RNA、マイクロRNA(miRNA)、タンパク質採取
- DNA/RNA/タンパク質検体のサイジング、定量、純度評価
- ハイスループットPCR、定量PCR(qPCR)、Sangerシークエンシング、マイクロアレイ、次世代シークエンシング(NGS)アッセイのためのカスタム分注および品質管理(QC)試験
- iWesternワークフロー、酵素結合免疫吸着測定法(ELISA)、QuantiGene Plexアッセイ
パセオンの細胞治療・遺伝子治療CDMOサービスをご紹介します。
各種資料
統合されたサプライチェーンと確かなグローバル調達戦略により、治験を強力に支援いたします。総合的な包装・流通サービス、文書作成サポート、最高水準の製品品質を誇るパセオンは、サプライチェーン全体のリスク低減に取り組んでいます。スイス、バーゼルの対照薬研究拠点Comparator Center of Excellenceは、英国、米国、アジアの戦略的拠点で活動する専門チームから構成されています。
対照薬調達サービスの内容
- 調達戦略のコンサルテーション、包括的な医薬品調達サービス
- 規制対応に関する高い専門知識・市場情報:頑健なサプライヤー適格性評価プロセスと偽造防止のための手順の整備による取引規制・税規制遵守の確保
- あらゆる対照薬、治験使用薬、標準製剤、プラセボ、レスキュー薬、基礎治療薬、併用薬、標準治療薬の調達
- サンプル1つから、数年間にわたって実施される第III相試験のための大量調達まで、開発企業からの直接調達や現地市場・自由市場からの調達によりあらゆるニーズに対応いたします。
- 治験サプライチェーンの最適化、ラベリング、包装、流通をカバーする総合的なサービス
各種資料
治験薬の盲検化およびcGMP・GAMP 5(自動化製造実践規範)に準拠した治験薬製造サービスでは、お客様のニーズに合わせてカスタマイズし、規制・品質ガイドライン遵守を確保した総合的なサービスを提供いたします。パセオンは非標準的で難易度の高いプロジェクトに日常的に取り組んでいるため、特別なプロセスニーズに対応できるツールや自動化技術の設計・製造経験を長年にわたって蓄積しています。
治験薬製造における標準的な盲検化サービスの内容
- プレフィルドシリンジ、バイアル、ボトル、点鼻スプレー、点滴静注バッグの盲検化
- グローバル調達した対照薬のブリスター包装・ボトルからの取り出し、印字除去、錠剤分割
- 単一品目/多品目オーバーカプセル化による完全自動盲検化
- プラセボおよび吸入用カプセルの高速製造
- 原薬や原薬混合物のマイクロドーズ投与、添加物の混合
- 危険化合物を用いる特殊プロジェクト
- 盲検化した対照薬の分析試験
治験資材の調達は、単に購入・入手することではありません。最適な機材・資材を速やかにかつ低価格で購入し、提供するには、治験のデザイン・要件の理解、世界各国の規制に関する深い知識、綿密な事前計画が必要です。パセオンの治験資材管理チームには、治験資材に関する高い専門知識だけでなく、サーモフィッシャーサイエンティフィックの幅広いラボ製品・臨床検査製品を利用できるという他にはない強みがあります。
- ラボ用装置・資材
- 医療/診断装置・資材
- 薬物送達装置・資材
- 一般的な治験資材
- 教育用資材
- 各治験デザインの必要に応じたその他資材
世界各国の規制の枠組みを常にモニタリングすることにより、法令を完全に遵守したサプライチェーンを確保しています[米国のAffordable Care Act(医療費負担適正化法)/Sunshine Act(情報公開法)など]。統一グローバル品質システムに基づき各種手順書を整備するとともに、すべてのサプライヤーの適格性を十分に確認することにより、しっかりとしたサプライチェーンを確保し、必要な治験資材を安定的に供給いたします。
各種資料
盲検化の維持や厳しい規制遵守を求められる治験薬のラベリングは、市販製品のラベリングに比べ高度な監視・管理を必要とします。高度な検査ソフトウェア、包括的な自社印刷技術、翻訳・薬事承認管理プロセス、ウェブベースの一元的な文書管理/ルーティングプラットフォームは、ラベリングサイクル時間全体の短縮を実現するために特別に設計されています。
ラベリングサービスの内容
- ラベルのセットアップ、翻訳作業の調整、薬事レビュー、無作為化のサポート
- 幅広いデザイン技術(お客様は英文原稿の提供のみ)
- ラベルのデザイン・製造と規制対応、包装、治験ロジスティクスを総合的にサポート
- 案件ごとの個別バッチ記録の管理・出荷
- 1パネル/2パネルラベル、GlobalPly多言語ブックレットラベル、DigiPlyデジタルブックレットラベル
- ATLASSM 翻訳管理システム
GlobalPlySM 多言語ブックレットラベルサービス
- GlobalPly多言語ブックレットラベルは、さまざまな表面材・粘着剤を用い、ほぼあらゆるサイズで製造することが可能です。
- 固定テキストおよび可変テキストの印刷を必要とするグローバル治験に適しています。
DigiPlySM デジタルブックレットラベルサービス
- DigiPlyは業界初のデジタル作成ラベルです。
- 治験国の追加/削除を週単位ではなく日単位で実施可能です。いったんデジタルでの作成・承認が完了すると、治験国追加時に新たなラベルデザインを作成する必要はありません。
- 合成二軸延伸ポリプロピレン(BOPP)を用いて作成されるDigiPlyラベルは、適用/使用時に破損することがないため、治験期間を通してデータの完全性を確保することが可能です。
- 100言語以上で最大60ページのラベルを作成でき、重要な記載事項の追加や大きな文字サイズ・さまざまな色の使用が可能です。
- 治験薬ラベルに特化したバリデーション済みのウェブベース翻訳・承認管理システムです。オンラインでのラベル発注、翻訳、承認からラベル製造まで管理します。
- 治験薬ラベル製造に要する時間を50%以上削減
- 各国の規制要件の保管・自動入力機能
- 承認を必要とする各国関連会社へのタスク翻訳のモニタリングと管理
- コンプライアンス不良ユーザーのエスカレーションプロセスと詳細なパフォーマンス指標の追跡
- 各国の規制情報を含む標準フレーズライブラリにより、翻訳作業の重複を排除できます。
各種資料
治験の成否はさまざまな要因に左右されますが、その1つが治験薬サプライチェーンの適切な計画・管理です。パセオンの治験サプライチェーン最適化サービスは、治験の初期戦略構築から被験者登録、実施、終了まで治験サプライチェーンを管理し、最適化・合理化を実現する総合的なサービスです。
患者さんの登録開始後は、必要な治験薬が必要な場所にあり、患者さんに確実に投与できるよう適切なサプライチェーン管理を維持しなければなりません。また、治験薬の供給予測、重要なステークホルダーとのコミュニケーション、成果指標の報告、再供給の計画、有効期限延長の調整なども適切なサプライチェーン管理には不可欠な要素です。
- 供給戦略構築のための積極的なサポート
- 高度なモンテカルロ・シミュレーション技術を用いた頑健な治験薬供給予測
- 治験の進捗に伴って得られる実際のデータを反映した継続的な予測調整
- 積極的なサプライチェーン管理により、必要に応じた治験薬・資材の的確な供給を確保することが可能です。
- サプライチェーンのリスク、無駄、複雑化を最小限に抑えます。
- 予算報告および予算管理ダッシュボード
- 開発のさまざまな段階における個々の治験、治験ポートフォリオ、医師主導治験(IIT)の管理
- 治験終了時の治験薬・資材の返却および数量管理により、治験実施計画書の要件への適合と適切な治験終了を確保
各種資料
パセオンファーマサービシズの自社施設により、室温/冷蔵/凍結包装技術を提供いたします。これら施設は世界の戦略的拠点に整備されており、各地域のニーズに的確に対応することが可能です。また、統合されたITシステムが各包装施設を結び、バーコード規格による在庫管理が可能です。
一次包装
- 温度・湿度管理下の製造室における冷間成形/熱成形ブリスター包装ライン
- 社内での金型製作、オンライン印刷、工程内視覚制御システム
- シングル包装/マルチ包装技術
- サイクル時間短縮を実現する自動化、高活性化合物/粉体化合物を独立した技術資源で取り扱う封じ込め設備
二次包装・ラベリング
- 小児用安全ブリスターシート/ボトル、バイアル、貼付剤、シリンジ、吸入器などのデバイスを含むさまざまな包装
- 温度管理下の製造室における手作業または自動での包装・組立て
- 1パネルラベル、マルチパネルラベル、1〜3パネルブックレットラベル、デジタルラベルのデザイン・翻訳・盲検化
ProSyriesSMプレフィルドシリンジの組立て・ラベリング
- 精密制御によるシリンジ製造では、温度管理下の完全自動連続プロセスにより組立て・ラベリングを実施
- 室温環境・低温環境のいずれにも対応できる自動製造ライン
- 光学式文字認識・検証(OCR-OCV)システムと品質を作り込むプロセスにより、可変テキスト/固定テキストの正確性および判読性を確認する全数検査を確保
- ProSyriesシリンジトレイは、航空輸送時のストッパー/プランジャーの移動を防ぐよう設計されています。
- ProSyries高精度シリンジラベリングでは、プレフィルドシリンジのあらゆる充填量を±0.5 mmの精度で表示することが可能です。
プレフィルドシリンジの組立て、一次・二次包装、治験薬のラベリング・盲検化、低温流通に特化したサーモフィッシャーサイエンティフィック研究拠点Center of Excellenceをご紹介します。
各種資料
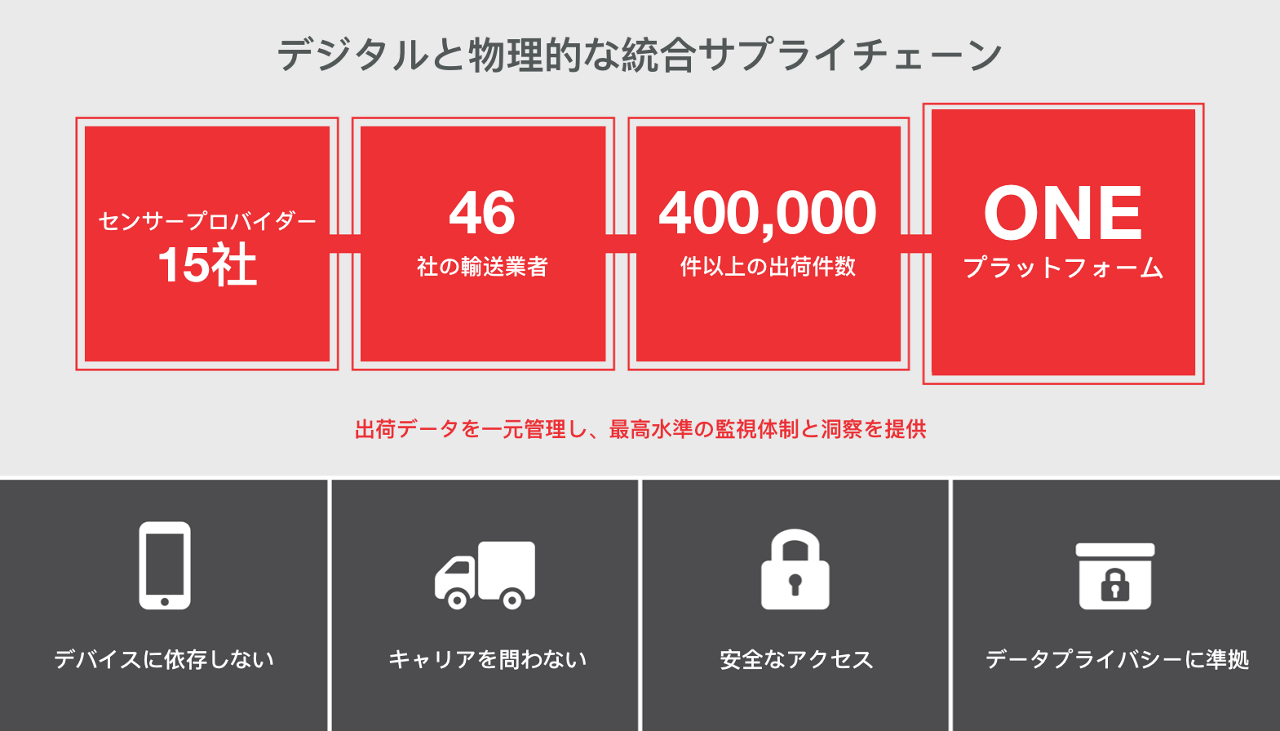
輸送状況の見える化により、私たちが暮らす世界をより健康で、より清潔、より安全な場所にするというお客様の取り組みを支援いたします。
グローバルな見える化:全世界の輸送状況をいつでも、どこからでも確認できます。
- すべての輸送品を一元的に追跡
- 輸送のあらゆる段階を常に把握できます。
- リアルタイムの温度・位置追跡機能により低温輸送品の輸送状況を見える化
- 遅れや逸脱の追跡
- 重要な低温輸送品・高額輸送品のための高度モニタリング
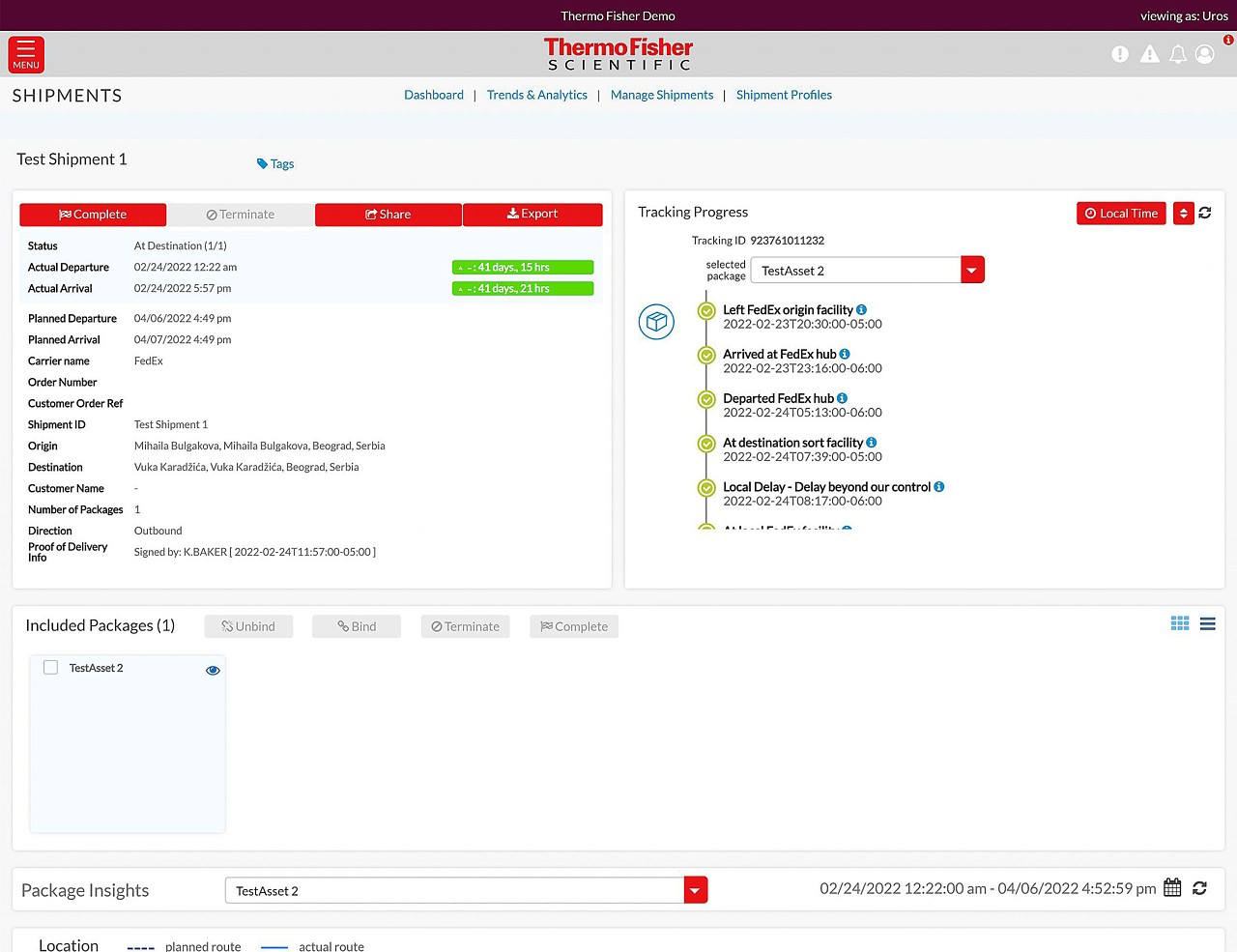
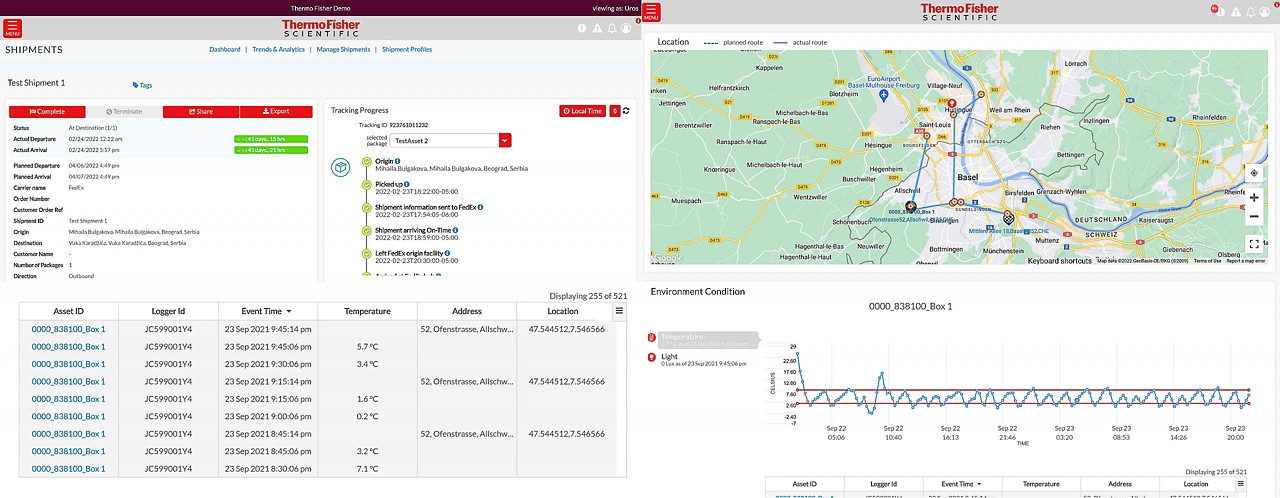
Track and Traceデジタルポータル
すべての輸送品を一元的に追跡
リアルタイムセンサー
リアルタイムの温度・位置追跡機能により低温輸送品の輸送状況を見える化
パセオンは、30年以上にわたって世界各国の製薬・バイオテクノロジー企業各社の治験薬供給・流通・ロジスティクスのニーズに対応してきた実績を誇ります。27拠点の専用GMP/GDP施設が、5大陸にまたがる38以上のパートナーデポと提携して提供するグローバル流通により、規制を遵守し、世界150ヵ国以上をカバーするサービスで、あらゆる適応症について的確な物流管理を確保することが可能です。ロジスティクスのエキスパートが、パセオンのネットワーク全体を通して、ラベリング/包装済みの室温/低温保管治験薬や対照薬、プラセボの保管・流通、輸入/輸出サービス(現時点で24ヵ国以上の輸入代行者業務を含む)、治験薬の返却・廃棄に的確に対応いたします。
総合輸送マネジメントサービスの内容
- 厳しい品質・パフォーマンス基準に基づきサプライヤーを評価するサプライヤー適格性評価
- 輸送方法の最適化(配送業者の選定・管理)により、お客様の目標に最適な輸送法方を提案
- 通関・規制対応に関する的確なサポートで円滑な作業を確保し、コストのかかる遅れのリスクを低減させます。
- 適切な低温輸送品管理、環境に配慮した再使用可能搬送容器プログラム
- 発送サービスでは、スケジュール設定・文書作成のあらゆる側面を調整いたします。
- 平日24時間体制の電話/メールサポートを含む積極的なTrack and Trace(追跡・トレーサビリティ)サービス
- すべての輸送品に関するデータの客観的モニタリング・報告により、事実に基づく意思決定を支援いたします。
- 請求書処理の一元化により、サプライヤー別インボイス管理の事務負担が軽減されます。
輸入者代行業務(IOR)
世界の戦略的拠点を結ぶcGMP施設ネットワーク、ロジスティクスに関する深い専門知識、そして各国のエキスパートが構成するチームを備えたサーモフィッシャーサイエンティフィックは、25ヵ国以上のIORサービスを含め、サプライチェーン全体を的確に管理できる独自のサービス力を誇ります。
IORとは、以下の事項に責任を負う法人です。
- 輸入品の各国法令遵守の確保
- 納税申告書および関連書類の提出
- 輸入品に対して課せられた輸入関税およびその他税金の支払い
- サーモフィッシャーサイエンティフィックは、厳しい審査を受け、各地域/国の法令を遵守したIORサービスを全世界で提供いたします。
グローバルロジスティクス・ヘルプデスク
私たちは、正しい患者さんに治験薬をスケジュール通りに完全な形で届けることの重要性を十分に理解しています。グローバルロジスティクス・ヘルプデスクは、治験サービスネットワークをご利用の全世界のお客様をサポートするチームです。輸送遅延時の積極的な介入・解決を含むTrack and Trace(追跡・トレーサビリティ)サービスや、輸送品に関する問い合わせに平日24時間体制で対応する電話・メールサポートを提供いたします。
グローバルヘルプデスクのサービス内容
- 輸送状況の追跡に関するお問い合わせ
- 輸送品の破損・取り違え
- 通関手続きに関する問題
- 輸送遅れ・紛失
- 温度逸脱に関する報告
ヘルプデスクへのお問い合わせは、
メール(GL.HD@thermofisher.com)または電話(本リストに掲載されている国別電話番号) でご連絡ください。ご連絡にあたっては、治験番号や梱包明細書をお手元にご用意ください。
各種資料
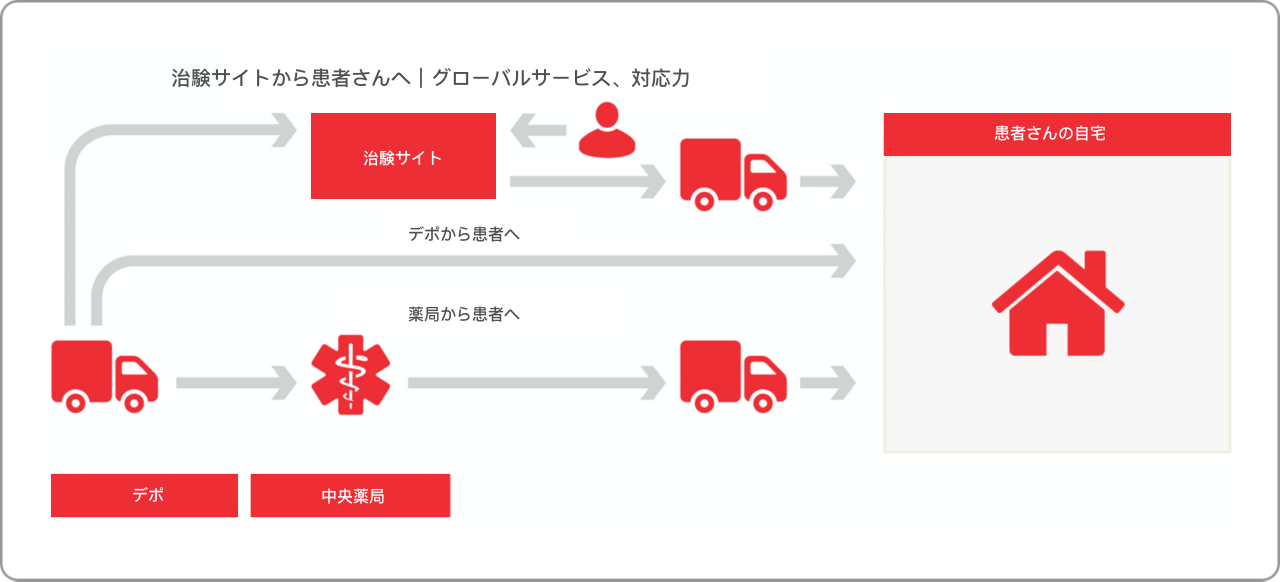
通常、治験では30%の患者さんが脱落し、リスクとコストの増加に直結します。患者さんによると、治験参加で最も負担に感じることの3つのうちの2つが、治験実施医療機関の所在地と来院に要する時間です。分散型臨床試験(DCT)では、このような負担を軽減または排除することが可能です。
DCTの歴史は1990年代に遡りますが、パセオンはDirect-to-Patient(患者さんへの直送)サービスを当初から提供しており、現在では50ヵ国以上をカバーしています。DCTは増加しつつありますが、治験依頼者だけでなく、治験実施医療機関や患者さんにとっての治験のやりやすさや準備状況はさまざまであるのが現状です。パセオンが従来型治験のほか、ハイブリッド型治験、100%分散型治験をサポートするサービスを提供しているのはこのためです。
- 個人情報保護の関連法令を遵守
- 50ヵ国以上をカバーするDirect-to-Patient(患者さんへの直送)サービスとともに、その他の地域における治験も業界最高のグローバルネットワークを駆使したサービスでサポートいたします。
- 全米50州をカバーする治験薬・非治験薬配送サービス
- スケジュール通りに治験薬/治験資材を患者さんの自宅に配送
- 患者さんや治験実施医療機関、また、必要に応じ在宅看護師との連携による配送手配
- 自動応答技術(IRT)を用いた治験およびIRTを用いない治験のためのソリューション
- 分散型臨床試験のセットアップ、患者さんの服薬遵守サポートに関する確かな専門知識
- CRO、IRTプロバイダー、治験サプライチェーン業務担当者、治験推進業務担当者が緊密に連携するチーム作業により、治験立ち上げ期間をわずか数ヵ月に短縮可能です。
スマート包装(アドヒアランス確認のためのソリューション)
DCTに加え、スマート包装技術により、患者さんの服薬アドヒアランス向上に貢献いたします。治験薬のスマート包装は、超小型回路を用いて投薬データを受動的に測定し、患者さんのスマートフォンなどを介してクラウドに送信する仕組みです。データを受信したクラウドベースのプラットフォームは、高度なアルゴリズムを用いて患者さんの服薬行動を分析し、何か問題があった場合には注意喚起のフラグを付与します。これにより治験実施医療機関スタッフは患者さんを「救援」し、正しい用法・用量に戻るよう指導することができます。
服薬アドヒアランス向上のためのスマート包装を用いることにより、信頼性の高い充実したデータセットを取得することができ、患者さんの服薬状況を把握しやすくなります。大部分の治験デザインで利用できる技術です。スマート包装は特に以下のような治験に有用です。
- 投与間隔の正確な把握が必要とされる治験
- 用法・用量が複雑な治験
- 治験期間が1年以上にわたる治験
- 治験実施医療機関への来院間隔が長い治験(分散型臨床試験)
各種資料
Global Gatewayポータルは、いつでも、どこからでもリアルタイムでデータを確認できる透明性を提供するポータルです。治験薬・治験資材の在庫や輸送 に関する情報を簡単に素早く確認することができる機能を製薬・バイオテクノロジー企業各社に提供いたします。